Development of protein hydrolysis approach to increase the nutritional benefits of freshly prepared meat and fish.
A scientific support paper by Dr Adrian Hewson-Hughes | Nutrition, Food Safety & Innovation Advisor, GA Pet Food Partners.


Introduction.
The animal protein content is well established as being the essence of premium quality foods for dogs and cats, and many pet owners recognise that the “quality over quantity” adage applies here. According to market research company Mintel, 59% of cat owners and 57% of dog owners say that the quality of meat is more important than the overall meat content in pet food (MINTEL, 2017).
GA Pet Food Partners has long recognised this. Since the introduction of Freshtrusion®, GA has led the way in developing and manufacturing diets containing increasing amounts of freshly prepared meat and fish protein sources. The benefits of using fresh meat & fish sources over dried, rendered meat & fish meals, including better taste and increased digestibility, are well appreciated by both pets and their owners.
Striving to offer our Partners even better products.
The pet food market is very dynamic and while highlighting the exact high proportion of meat/fish/poultry in products is a wise move, this is becoming a base expectation rather than the desired quality for these products from pet owners. Here at GA, we are constantly striving for ways to offer Partners even better products, and that is why we set out to do the seemingly impossible – come up with a way to cook our fresh meat and fish ingredients to make them even better for pets.
The idea is to increase the nutritional value of the protein within our fresh meat and fish ingredients by converting the protein into small peptides, which are more easily absorbed by the pets eating it (we are calling it ‘HDP’ – Highly Digestible Protein). To help us in this quest, we identified experts at Nofima, a leading research institute for applied food research-based in Norway, to optimise conditions for enzymatic digestion of selected meat and fish raw materials and analyse them to demonstrate we could achieve what we wanted.


Protein Digestion – aka Proteolysis or Hydrolysis
Proteins are large molecules made up of individual ‘building blocks’ called amino acids. After eating food containing protein, the process of proteolysis begins as enzymes released in different parts of the gastrointestinal tract break it down into amino acids and small peptides. This enables these building blocks to be absorbed into the body, where they can be recombined to build new proteins (such as muscle, skin, hair, antibodies, enzymes, hormones, etc.).
It is also possible for protein sources to undergo a controlled enzymatic proteolysis process as part of their preparation for inclusion in manufactured foods and nutritional products. For example, protein hydrolysates have been used for decades in human nutrition, most notably in the production of hypoallergenic infant milk formulas for babies/children allergic to cow’s milk protein.

Enzymatic or Chemical Hydrolysis
Protein hydrolysis – the breaking of peptide bonds that join amino acids together through the addition of water – can be achieved by different methods: chemically using acids or bases (alkaline) or enzymatically (the approach we are focusing on). While the methods of acid and alkaline hydrolysis of proteins offer the advantage of low cost, there are negative consequences in terms of the nutritional quality of the hydrolysates produced. Acid hydrolysis results in complete destruction of the essential amino acid tryptophan, as well as partial loss of methionine, cystine and cysteine (Pasupuleki & Braun, 2010). Similarly, alkaline hydrolysis results in the complete destruction of most amino acids, although tryptophan can survive intact (Dai, et al., 2014), (Hou, et al., 2017).
Compared to acid and alkaline hydrolysis, the main advantages of enzymatic hydrolysis of proteins are:
- The hydrolysis conditions such as temperature and pH are mild and do not result in any known loss of amino acids.
- The use of protease enzyme(s) is more specific and precise in controlling the extent of hydrolysis and the size of peptides.
- The small amounts of enzyme used can be easily deactivated (e.g., heating to 80 – 85ºC for at least 3 minutes) to halt the hydrolysis reaction. (Hou, et al., 2017).


Nutritional benefits of enzymatically hydrolysed protein: protein digestibility and absorption.
In addition to the method of protein hydrolysis used, as outlined previously, the nutritional value of protein hydrolysates depends on the composition of free amino acids, small peptides (typically di- and tri-peptides) and large peptides present. Historically it was believed that only free amino acids were absorbed from the gastrointestinal tract by specific amino acid transporters. This does occur, but it is now recognised that the majority of amino acids are absorbed as di- and tri-peptides by the broad-specificity peptide transporter PepT1 (Fei, et al., 1994). PepT1 can potentially transport all 400 di-peptides and 8,000 tri-peptides that result from combining the 20 different dietary amino acids (Daniel, 2004). Therefore it would be expected that ingestion of a protein hydrolysate containing high proportions of di- and tri-peptides would facilitate protein digestion and absorption, resulting in increased digestibility and amino acid bioavailability.
Clearly, establishing the best enzyme and hydrolysis conditions is critical to be able to create protein hydrolysates with the desired final peptide size profiles. Peptide size distribution can be determined using a technique called size exclusion chromatography. Size exclusion chromatography (SEC) is an analytical chemistry technique in which mixtures of molecules (such as proteins or peptides) dissolved in a solution are separated by their size (as outlined in figure 1).
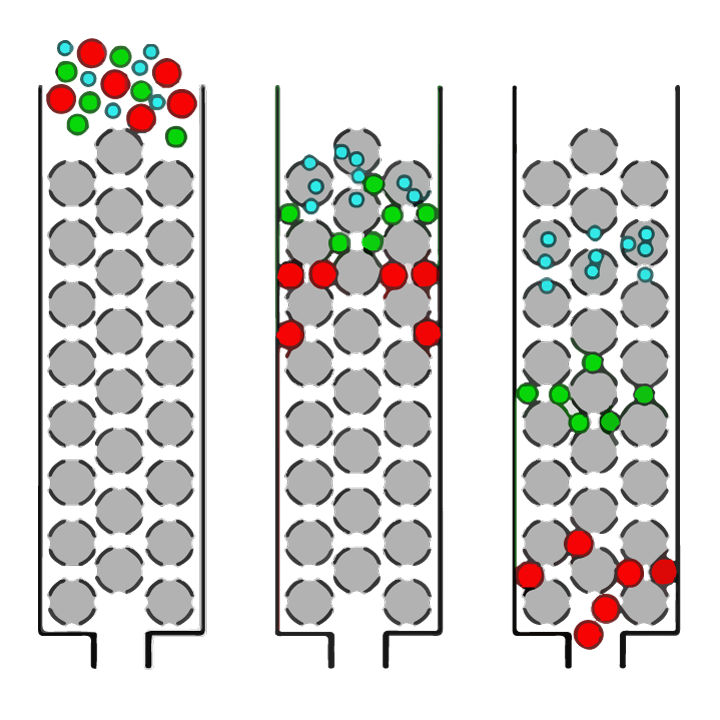
FIGURE 1. Simple overview of the separation of molecules of different sizes in a solution by size exclusion chromatography (SEC). The solution is applied to a column which is packed with a resin of porous spherical beads (grey spheres). Large molecules (red circles) will not be able to enter into the pores (holes) of the beads and therefore pass down the column relatively quickly and will be detected first. Smaller molecules within the sample can enter the pores to varying degrees depending on their size. ‘Medium-sized’ molecules (green circles) will be able to enter some beads but not others and so will take longer to pass through the column, while the smallest molecules (blue circles) will be able to enter all the pores and will take the longest to pass through the column.
Methods
Raw Materials – Fresh samples of chicken carcass, duck carcass and salmon frames were size-reduced, homogenised into a thick paste and frozen. Fresh lamb liver was frozen whole. The materials were sent to Nofima, Ås, Norway, for proteolysis and analysis.
Proteolysis – For each raw material (chicken, duck, salmon and lamb), a 500g sample was mixed with 990ml distilled water in a glass reaction vessel and stirred at 300rpm. For each raw material, three different protease enzymes were tested at two different concentrations and two time-points resulting in 48 hydrolysate samples for analysis.
Size Exclusion Chromatography – The molecular weight distribution of the water-soluble protein fraction of the hydrolysates was determined by size exclusion chromatography using a Shimadzu LC-20AT high-performance liquid chromatography (HPLC) system with a photodiode array detector (SPD M20A) set at 214nm.
Collagen Peptide Content – Hydroxyproline is a modified amino acid, the presence of which is mainly confined to collagen. Hydroxyproline content in protein hydrolysates can be used as an indirect measure of the amount of collagen/collagen peptides present. A full amino acid analysis (including hydroxyproline) of each raw material was carried out by Nofima Biolab; in addition, hydroxyproline content was determined at an accredited laboratory (ALS, Norway) in the water-soluble fraction of the hydrolysates.


Results
Peptide Size Distribution of Hydrolysates – In general, for each enzyme tested, incubation of each raw material with the higher concentration of enzyme and for a longer duration resulted in a ‘beneficial’ shift in the peptide size profile of the hydrolysates (i.e. an increase in the proportion of smaller peptides). This is highlighted in Figure 2, which shows results for each raw material using the ‘best’ enzyme at ‘non-optimal’ concentration and duration compared to ‘optimal’ concentration and duration. With optimised conditions, we found that 100% of peptides were ≤3 kDa, and more than 75% were < 0.5 kDa (Figure 2).
Based on the collective evidence of several studies in a number of species (e.g. rat, pig, dog, human; see (Zhangi & Matthews, 2010) for an overview, it is generally well accepted that:
- Absorption of peptides is better compared to intact protein.
- Absorption of peptides is better than free amino acids.
- Absorption of small peptides is better than of large peptides.
Physiologically, the majority of amino acids are absorbed as small peptides consisting of 2 or 3 amino acids joined together (di- and tri-peptides, respectively). Therefore it would be expected that ingestion of a protein hydrolysate containing high proportions of di- and tri-peptides would facilitate protein digestion and absorption, resulting in increased digestibility and amino acid bioavailability. The average molecular weight of an amino acid is 110 Daltons (Da), so di- and tri-peptides would have a molecular weight of approximately 220-330 Da (0.2-0.3 kDa). Our results in achieving protein hydrolysates with more than 75% of peptides smaller than 0.5 kDa (i.e. up to ~ 5 amino acids) means the protein in our kibbles would be highly digestible and easily absorbed by the pets eating it. It is planned to demonstrate this through a feeding study in collaboration with the Faculty of Veterinary Medicine, University of Ghent.
In addition, achieving 100% of peptides of 3 kDa or smaller decreases the risk of triggering an allergic reaction to the protein sources and can therefore be considered hypoallergenic.
Figure 2.
Size distribution (kDa) of peptides in the water phase of hydrolysates of each raw material incubated with the same enzyme under ‘non-optimised’ and ‘optimised’ conditions in terms of enzyme concentration and duration of hydrolysis. Note in particular how the percentage of peptides between 1.0-3.0 kDa decreases and peptides <0.5 kDa increases, moving from ‘non-optimised’ to ‘optimised’ conditions.

Collagen Peptide Content
For each raw material tested, enzymes A and C performed ‘better’ in general (in terms of recovering a greater percentage of hydroxyproline in the water phase of the hydrolysates) than enzyme B when comparing a given duration of hydrolysis and enzyme concentration (e.g. see results for salmon in figure 3).
Since ‘intact’ collagen protein is not soluble in water, the presence of hydroxyproline (our marker of ‘collagen’) in the water phase indicates that the collagen protein has been digested into collagen peptides (which are water-soluble). Our results illustrate that we are able to use enzymatic proteolysis to create raw materials that are able to bring potential functional benefits such as supporting joint health, skin health, and gut health through the collagen peptides present within them.

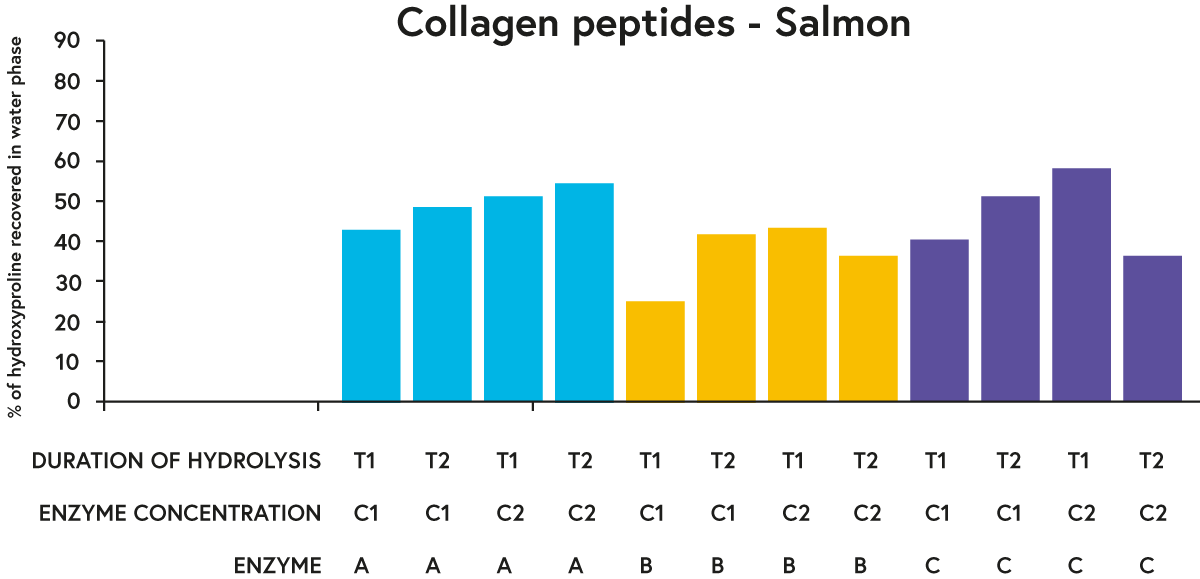
FIGURE 3. Percentage of hydroxyproline (an amino acid found almost exclusively in collagen) recovered in the water phase of hydrolysed salmon with three different enzymes (A, B or C) incubated with the raw material (salmon) at two different concentrations (C1 or C2) for two different time periods (T1 or T2).

Conclusion
These positive results present opportunities to gain extra value from the natural presence of collagen within certain raw materials by creating collagen peptides with the potential to deliver functional benefits such as maintaining healthy joints in active pets and improving mobility & flexibility of joints in older pets, for example.
With the high percentage (>75%) of small peptides (<0.5kDa) produced under ‘optimised’ conditions based on this research, the first part of our HDP goal is achieved. The next important step is to demonstrate that kibble made with this HDP is indeed more digestible and bio-available than our existing freshly prepared products – we are busy performing this in a feeding study with the University of Ghent Vet school. Watch this space!



References
- Cave, N., 2006. Hydrolyzed protein diets for dogs and cats. Veterinary Clinics Small Animal Practice, Volume 36, pp. 1251-1268.
- Dai, Z., Wu, Z., Jia, S. & Wu, G., 2014. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection.. J Chromatography B, Volume 964, pp. 116-127.
- Daniel, H., 2004. Molecular and integrative physiology of intestinal peptide transport.. Annual Review of Physiology, Volume 66, pp. 361-384.
- Fei, Y. et al., 1994. Expression cloning of a mammalian proton-coupled oligopeptide transporter.. Nature, Volume 7, pp. 563-566.
- Hanaoka, K. et al., 2019. Characterization of proteins and peptides molecular weight during the manufacturing of pet food palatants. [Online] Available at: https://www.diana-petfood.com/emea-en/publications/
- Hou, Y. et al., 2017. Protein hydroysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. Journal of Animal Science and Biotechnology, pp. 24-36.
- Knights, R., 1985. Processing and evaluation of protein hydrolysates. In: Nutrition for Special Needs. New York: Marcel Dekker, pp. 105-115.
- MINTEL, 2017. Greater transparency in terms of protein in pet food, s.l.: MINTEL REPORTS.
- Pasupuleki, V K, Braun, S, 2010. State of the art manufacturing of protein hydrolysates. In: Protein Hydrolysates in Biotechnology. New York: Springer, pp. 11-32.
- Zhangi, B. & Matthews, J., 2010. Physiological importance and mechanisms of protein hydrolysate absorption. In: Protein Hydrolysates in Biotechnology. New York: Springer, pp. 135-177.
