Freshtrusion® – creating the world’s finest pet food.
A scientific support paper by Dr Adrian Hewson-Hughes | Nutrition, Food Safety & Innovation Advisor, GA Pet Food Partners.


It starts with high-quality raw materials.
At GA, we aim to make the world’s finest pet food. In order to help us deliver this, we developed a unique process which we called Freshtrusion®. The process uses technology that allows us to provide pets with delicious, highly nutritious diets by incorporating freshly prepared meat and fish ingredients into our kibbles.
The Freshtrusion® journey begins with the high-quality fresh, chilled meat and fish raw materials that we collect at the source and transport back to our manufacturing site in Lancashire in our own refrigerated lorries to maintain the freshness.
Gently cooked in our Meat Kitchen.
Since the introduction of Freshtrusion®, we have hailed our gentle cooking process as the best way to ‘protect the proteins’ in our Freshly Prepared meats and fish and ensure the best digestibility and nutritional value of the kibbles for the pets that eat them.
Research has demonstrated that ‘thermal processing’ (cooking) can alter the digestibility and bioavailability of proteins in meat (outlined in Figure 1). In particular, cooking at lower temperatures can induce structural/conformational changes (e.g. ‘unfolding’) in the meat proteins, which exposes more cleavage sites that the digestive protease enzymes in the gastrointestinal tract can act on to break down the protein, leading to improved protein digestibility (Bhat et al., 2021).
In contrast, high-temperature cooking can induce extensive protein oxidation, forming different kinds of cross-links and protein aggregation, making it harder for digestive enzymes to break down the protein and therefore decreasing protein digestibility (Bhat et al., 2021).
In beef muscle cooked at 100oC, protein oxidation increased gradually over the duration of the 45-minute cooking time; the effect of rapid, high-temperature cooking (1 minute at 270oC, reaching a maximum core meat temperature of 170oC) on protein oxidation was similar to cooking for 30 minutes at 100oC (Santé-Lhoutellier et al., 2008). Protein aggregation increased dramatically after 5 minutes of cooking at 100oC and remained at a similar level after 45 minutes, with a similar increase induced by cooking for 1 minute at 270oC. Associated with these biochemical changes, a 42% decrease of in vitro digestion rate by pepsin (a protease enzyme from the stomach responsible for the first stage of protein digestion) was seen after only 5 minutes of cooking at 100oC and a 58% decrease was seen at 45 minutes. Cooking for 1 minute at 270oC induced the same decrease in pepsin activity as 30 minutes of cooking at 100oC. These decreases in pepsin digestibility rate were found to be significantly correlated with increased protein oxidation and protein aggregation. (Santé-Lhoutellier et al., 2008).


Gently cooking – longer and lower.
Looking at a lower cooking temperature, pork muscle protein cooked at 70ºC for 30 minutes had significantly lower carbonyl levels (an indicator of protein oxidation) compared to pork cooked at 100ºC for 30 minutes.
Increasing the temperature to 140ºC for 30 minutes resulted in a further significant increase in protein oxidation (Bax et al., 2012). The rate of in vitro digestion by pepsin was increased for pork protein cooked at 70ºC for 30 minutes, whereas cooking at 100ºC and 140ºC decreased the rate of digestion due to oxidation-related protein aggregation (Bax et al., 2012).
Switching from in vitro (i.e. carried out in a ‘test tube’) to in vivo (carried out in a living organism), a study performed in minipigs measured the concentration of essential amino acids appearing in the blood following a meal of beef protein which had been cooked at 60ºC, 75ºC or 95ºC for 30 minutes (Bax et al., 2013).
The rate at which the amino acids are absorbed into the blood during the first 3 hours after eating the food is a good index of the speed of digestion of the protein.

Greater results
The results showed a more rapid increase in plasma amino acid levels after eating meat cooked at 75ºC compared to 95ºC (and meat cooked at 60ºC was in between), suggesting an increased speed of digestion when the cooking temperature was increased from 60ºC to 75ºC and a decrease in the speed of digestion when the temperature was increased from 75ºC to 95ºC (Bax et al., 2013).
These results are in line with the in vitro effects described above, suggesting that cooking the meat at 75ºC resulted in the ‘unfolding’ of the protein structure, making it easier for the enzymes in the digestive tract of the minipigs to digest the protein and absorb the amino acids whereas increasing cooking temperature leads to protein oxidation and aggregation making it harder for the protein to be broken down by digestive enzymes.

Native Protein
Gentle Cooking
Protein Unfolding

Increased exposure of hydrolytic sites to digestive enzymes
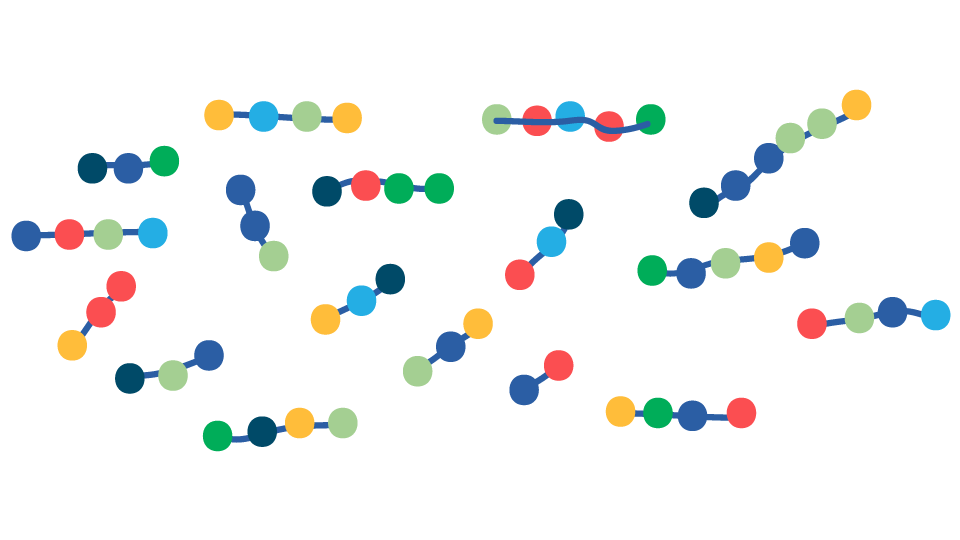
Improved Protein Digestibility
FIGURE 1. Overview of the effects of temperature on animal proteins during cooking and consequences for gastrointestinal (GI) digestion following consumption. Low-temperature cooking leads to the unfolding of proteins, exposing multiple sites where protease enzymes (shown as scissors) in the GI tract can gain easy access to digest the protein. Severe oxidation and protein aggregation caused by high-temperature cooking alters the protein structure making enzyme cleavage sites less accessible to digestive enzymes and, therefore, more difficult to digest.

Native Protein
High Temperature Cooking

Protein Aggregation & Oxidation
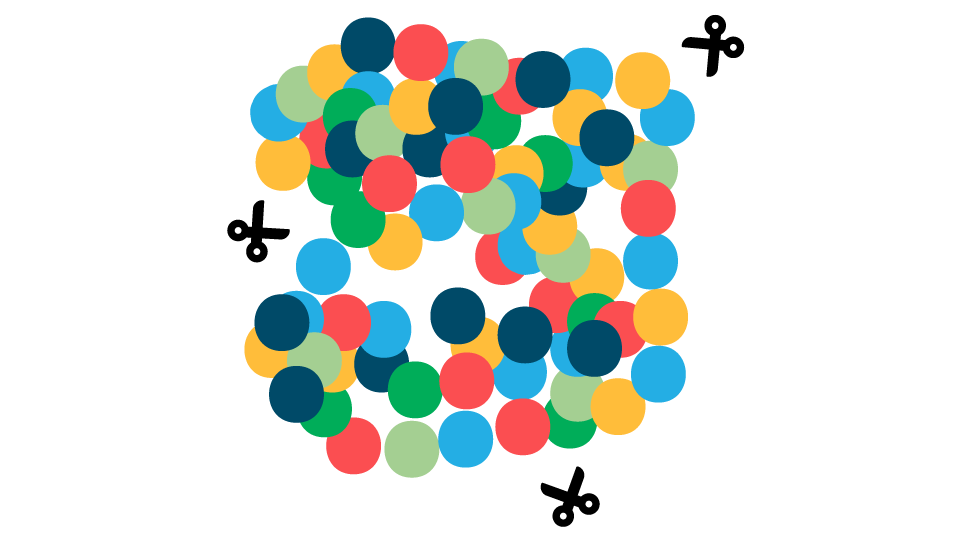
Decreased exposure of hydrolytic sites to digestive enzymes
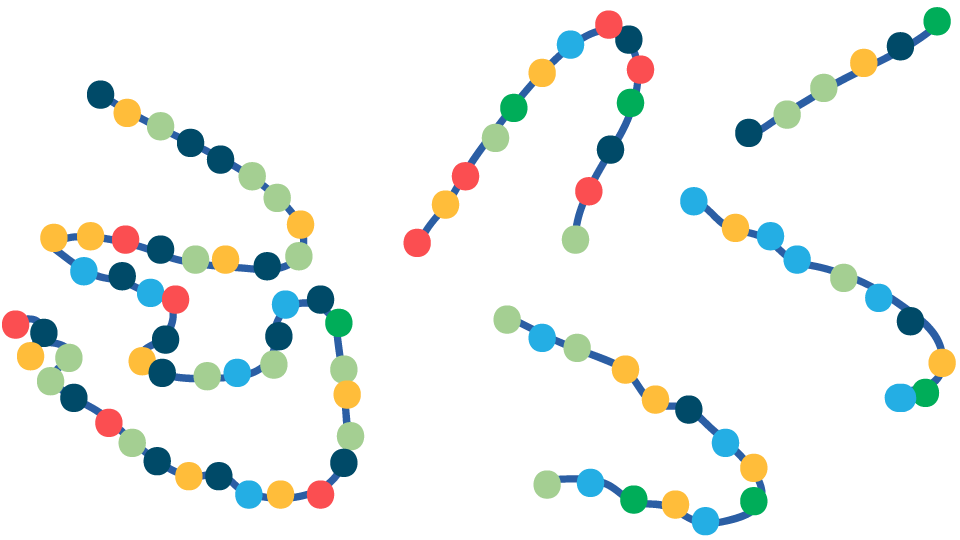
Decreased Protein Digestibility
Highly Nutritious Kibbles
After cooking in the Meat Kitchen, the freshly prepared meat/fish is combined with the dry ingredients in the recipe and extruded to create dry dog (and cat) foods. It has been shown that dry dog foods made with fresh meat or fish materials as the only animal protein source (diets made with either beef loin, pork loin, chicken breast, salmon fillet or pollock fillet) resulted in very high protein digestibility values (Faber et al., 2010). Average protein digestibility values from adult dogs fed each diet were 89.7% (beef), 90.5% (pork), 88.9% (chicken), 90.5% (pollock) and 89.2% (salmon).
In another study, where dry diets made with fresh meat or rendered meat meal were compared, it was found that the fresh poultry diet had significantly higher ileal protein digestibility (83%) than the poultry meal diet (74%) when fed to adult dogs (Murray et al., 1998). In the same study, a similarly high ileal protein digestibility was reported for a fresh beef diet (80.4%), and a similar result was seen for kibbles made with a rendered beef meal (79.9%), indicating the digestibility of different meat meals can be variable. In another study, the protein digestibility of a poultry meal-based dry dog food was 80.3% (Tjernsbekk et al., 2017). When a proportion of the poultry meal was partially replaced with raw chicken meat the protein digestibility of the diet was 81.3% (Tjernsbekk et al., 2017).
In contrast, high-temperature cooking can induce extensive protein oxidation, forming different kinds of cross-links and protein aggregation, making it harder for digestive enzymes to break down the protein and therefore decreasing protein digestibility (Bhat et al., 2021).
A study was performed on a GA diet containing 55% Freshly Prepared poultry and fish ingredients plus 23% chicken meal (Brierley, 2019). Pepsin digestibility of the kibbles tested in vitro was 91% and the in vivo protein digestibility following a feeding trial in 10 dogs was 80.3% (N.B. it is not unusual for in vivo protein digestibility to be lower than in vitro values, Biagi et al., 2016).

Benefits of Freshtrusion®
Collectively, the above studies indicate that our low-temperature cooking conditions not only protect proteins from severe oxidation and aggregation but can induce favourable changes that enhance their digestibility. Incorporating these high-quality meat and fish ingredients into our dry pet foods results in diets with exceptional digestibility and nutritional value for the pets.
References
- Bax, M-L., Aubry, L., Ferreira, C., Daudin, J-D., Gatellier, P., Rémond, D., Santé-Lhoutellier, V. Cooking temperature is a key determinant of in vitro meat protein digestion rate: Investigation of underlying mechanisms. J. Agric. Food Chem. 2012, 60: 2569-2576.
- Bax, M-L., Buffière, C., Hafnaoui, N., Gaudichon, C., Savary-Auzeloux, I., Dardevet, D., Santé-Lhoutellier, V., Rémond, D. Effects of meat cooking, and of ingested amount, on protein digestion speed and entry of residual proteins into the colon: A study in minipigs. PLoS ONE 8(4): e61252. DOI: 10.1371/journal.pone.0061252
- Bhat, Z.F., Morton, J.D., Bekhit, A.E-D.A., Kumar, S., Bhat, H.F. Thermal processing implications on the digestibility of meat, fish and seafood proteins. Compr. Rev. Food Sci. Food Saf. 2021, 1-38. DOI: 10.1111/1541-4337.12802
- Biagi, G., Cipollini, I., Grandi, M., Pinna, C., Vecchiato, C.G., Zaghini, G. A new in vitro method to evaluate digestibility of commercial diets for dogs. Italian J. Anim. Sci. 2016, 15(4): 617-625. DOI: 10.1080/1828051X.2016.1222242
- Brierley, V. The effect of kibble density on the digestibility and palatability of dry extruded dog food. GA Pet Food Partners Internal R&D report, 2019.
- Faber, T.A., Bechtel, P.J., Hernot, D.C., Parsons, C.M., Swanson, K.S., Smiley, S., Fahey Jr, G.C. Protein digestibility evaluations of meat and fish substrates using laboratory, avian, and illealy cannulated dog assays. J. Anim. Sci. 2010, 88: 1421-1432. DOI: 10.2527/jas.2009-2140
- Murray, S.M., Patil, A.R., Fahey Jr, G.C., Merchen, N.R., Hughes, D.M. Raw and rendered animal by-products as ingredients in dog diets. J. Nutr. 1998, 128: 2812S-2815S.
- Santé-Lhoutellier, V., Astruc, T., Marinova, P., Greve, E., Gatellier, P. Effect of meat cooking on physicochemical state and in vitro digestibility of myofibrillar proteins. J. Agric. Food Chem. 2008, 56: 1488-1494.
- Tjernsbekk, M.T., Tauson, A-H., Kraugerud, O.F., Ahlstrøm, Ø. Raw mechanically separated chicken meat and salmon protein hydrolysate as protein sources in extruded dog food: effect on protein and amino acid digestibility. J. Anim. Physiol. Anim. Nutr. 2017, 101: e323-e331. DOI: 10.1111/jpn.12608
